вторник, 30 ноября 2010 г.
Cediranib
Name: 4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline
Alternate Name/Synonyms: AZD2171, Recentin
CAS Number: 288383-20-0
Molecular Formula: C₂₅H₂₇FN₄O₃
Molecular Weight: 450.51
Description: Cediranib (AZD2171) is a tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3, c-KIT, and platelet-derived growth factor receptors. Studies show cediranib to be generally well tolerated as monotherapy at doses of 45 mg/d or less.
Alternate Name/Synonyms: AZD2171, Recentin
CAS Number: 288383-20-0
Molecular Formula: C₂₅H₂₇FN₄O₃
Molecular Weight: 450.51
Description: Cediranib (AZD2171) is a tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3, c-KIT, and platelet-derived growth factor receptors. Studies show cediranib to be generally well tolerated as monotherapy at doses of 45 mg/d or less.
понедельник, 29 ноября 2010 г.
воскресенье, 28 ноября 2010 г.
суббота, 27 ноября 2010 г.
Gefinitib
Name:N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine
COC1=CC2=C(C=C1OCCCN1CCOCC1)C(NC1=CC(Cl)=C(F)C=C1)=NC=N2
MW 446.902
C22H24ClFN4O3
References:
1. Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G: Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000 May;6(5):2053-63
2. Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J: Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001 Sep 1;61(17):6500-10.
3. Moasser MM, Basso A, Averbuch SD, Rosen N: The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001 Oct 1;61(19):7184-8.
4. Arteaga CL, Johnson DH: Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001 Nov;13(6):491-8.
5. Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5.
1. Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G: Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000 May;6(5):2053-63
2. Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J: Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001 Sep 1;61(17):6500-10.
3. Moasser MM, Basso A, Averbuch SD, Rosen N: The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001 Oct 1;61(19):7184-8.
4. Arteaga CL, Johnson DH: Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001 Nov;13(6):491-8.
5. Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5.
четверг, 25 ноября 2010 г.
Synthesis of Gefinitib
D'Souza, N., Castañer, J., Levin, M., Iressa. Drugs Fut 2002, 27, 4, 339.
Gibson K.H., Quinazoline derivs.. EP 0823900; JP 1999504033; US 5770599; WO 9633980 .
Gibson K.H., Grundy W., Barker A.J. et al.; Studies leading to the identification of ZD1839 (Iressa): An orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer. Bioorg Med Chem Lett 2001, 11, 14, 1911.
Molecules 2006, 11, 286-297
Bosutinib
Inhibitors of the BCR-ABL tyrosine kinase
Wikipedia
Synthesis of Bosutinib from 3-Methoxy-4-hydroxybenzoic Acid
понедельник, 22 ноября 2010 г.
Foretinib
Foretinib (synonim - GSK1363089) is a new MET and VEGFR2/KDR inhibitor (IC50 of 0.4 and 0.8 nM,respectively. Foretinib inhibits HGF receptor family tyrosine kinases - Ron (IC50 of 3 nM). Foretinib inhibits KDR, Flt-1, Flt-4 and KDR (IC50 of 6.8,2.8 nM and 0.9, respectively). In addition, foretinib inhibits members of PDGF family and the angiopoietin-1 receptor Tie-2. Foretinib exhibits modest activity against FGFR-1 and epidermal GFR and is inactive against 50 serinе/threonine kinases, including cyclin-dеpendent kinasеs and protеin kinase C isoforms. To dеlinеate the cеllular effect of foretinib, VEGF - inducеd extracellular signal- regulated kinasе phosphorylation was used to assess the effect of the compound on phosphorylation of KDR in umbilical vein endothelial cells that resulted in an IC50 of 16 nM. Foretinib is the first orally available inhibitor of Met. Anticancer activity has been observed and Foretinib may represent for patients with renal-cell carcinoma.
Anti-Cancer Agents in Medicinal Chemistry, 2010, 10, 7-27
oretinib (GSK1363089, XL880) является новмй мультикиназным МЕТ и VEGFR2/KDR ингибитором (IC50 0.4 и 0.8 нМ, соответственно). Foretinib ингибирует HGF рецепторов тирозинкиназы HGF (IC50 3 нм для Rон). Foretinib ингибирует Flt-1, Flt-4 и KDR (IC50 6.8, 2.8 b 0.9 нм соответственно). Кроме того, он ингибирует семейство тромбоцитарного рецептор фактора роста и ангиопоэтина-1 рецептор Tie-2. Foretinib имеет небольшую активность в отношении фактора роста фибробластов рецептора 1 и рецептора эпидермального фактора роста и неактивен в отношении 50 серин/треонин киназ, в том числе циклин-зависимых киназ и протеинкиназы С изоформ.
Foretinib является первым представителем малых молекул ингибиторов МET. Противораковая активность Foretinib может использована для лечения пациентов с диагнозом почечно-клеточный рак.
Foretinib is a small-molecule inhibitor of HGF and VEGF receptor
tyrosine kinases with single-digit nanomolar IC50 values. It also
inhibits KIT, Flt-3, PDGFR-beta and Tie-2. Foretinib exerted
cytotoxicity against a broad panel of cancer cell lines. It also reduced
tumor cell migration, invasion, and tumor-induced angiogenesis.
catalogue number: F166
synonyms: EXEL-2880, XL880, GSK1363089
CAS: 849217-64-7
MW: 632.65 g/mol
synonyms: EXEL-2880, XL880, GSK1363089
CAS: 849217-64-7
MW: 632.65 g/mol
References
Cancer Res. 2009 Oct 15;69(20):8009-16.
Structure of the kinase domain of c-Met/HGFR bound to foretinib PDB: 3LQ8
Cancer Res. 2009 Oct 15;69(20):8009-16.
Structure of the kinase domain of c-Met/HGFR bound to foretinib PDB: 3LQ8
суббота, 20 ноября 2010 г.
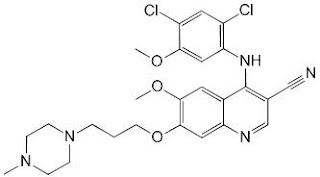
Dovitinib
“Molecularly Targeted Agents for Renal Cell Carcinoma: The Next Generation”, C. Lance Cowey and Thomas E. Hutson -Clinical Advances in Hematology & Oncology, 2010, 8, 357.
Lee S. H.; Lopes de Menezes, D. Vora, J. Harris, A.; Ye, H. Nordahl, L.; Garrett, E.; Samara, E.; Aukerman, S. L.; Gelb, A. B. Heise, C. In Vivo Target Modulation and Biological Activity of CHIR-258, a Multitargeted Growth Factor Receptor Kinase Inhibitor, in Colon Cancer Models. Clin. Cancer Res. 2005, 11 (10), 3633–3641.
Lopes de Menezes, D. E.; Peng, J.; Garrett, E. N.; Louie, S. G.; Lee, S. H.; Wiesmann, M.; Tang, Y.; Shephard, L.; Goldbeck, C.; Oei, Y.; Ye, H.; Aukerman, S. L.; Heise, C. CHIR-258: A Potent Inhibitor of FLT3 Kinase in Experimental Tumor Xenograft Models of Human Acute Myelogenous Leukemia. Clin. Cancer Res. 2005, 11 (14), 5281–5291.
Trudel, S.; Li, Z. H.; Wei, E.; Wiesmann, M.; Chang, H.; Chen, C.; Reece, D.; Heise, C.; Stewart, A. K. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood 2005, 105 (7), 2941–2948.
Trudel, S.; Li, Z. H.; Wei, E.; Wiesmann, M.; Chang, H.; Chen, C.; Reece, D.; Heise, C.; Stewart, A. K. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood 2005, 105 (7), 2941–2948.
Synthesis of Dovitinib
Tetrahedron Letters 47 (2006) 657–660
LHMDS mediated tandem acylation–cyclization of 2-aminobenzenecarbonitriles with 2-benzymidazol-2-yl acetates: a short and efficient route to the synthesis of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones
William R. Antonios-McCrea, Kelly A. Frazier, Elisa M. Jazan, Timothy D. Machajewski, Christopher M. McBride, Sabina Pecchi, Paul A. Renhowe, Cynthia M. Shafer and Clarke Taylor
LHMDS mediated tandem acylation–cyclization of 2-aminobenzenecarbonitriles with 2-benzymidazol-2-yl acetates: a short and efficient route to the synthesis of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones
William R. Antonios-McCrea, Kelly A. Frazier, Elisa M. Jazan, Timothy D. Machajewski, Christopher M. McBride, Sabina Pecchi, Paul A. Renhowe, Cynthia M. Shafer and Clarke Taylor
Axitinib
Synthesis of tert-butyl (1H-benzo[d]imidazol-2-yl)methylcarbamate.
tert-Butyl (1H-benzo[d]imidazol-2-yl)methylcarbamate.
Benzene-1, 2-diamine (1.05 g, 9.72 mmol) and N-(tert-butoxycarbonyl)glycine (1.71 g, 9.72 mmol) were dissolved in 30 ml of THF and cooled to 0 °C. Into the above solution was added N, N'-dicyclohexylcarbodiimide (2.41 g, 11.7 mmol ) in batches and the mixture was stirred at 0 °C for half an hour and then at room temperature overnight. The reaction mixture was filtrated and evaporated to afford a brown oil, which was purified by a silica-gel column chromatography (dichloromethane / methanol, 25:1 by volume) to get a yellow solid (2.24 g). The solid was dissolved in 20 ml of acetic acid and the solution was stirred at 72 °C for 8 h. The acetic acid was removed under reduced pressure and the crude compound was purified by a silica-gel column chromatography (dichloromethane / methanol, 25:1 by volume) to afford a white solid. Yield: 82%. m.p. 181-183 °C. IR (KBr) cm-1: 3343 (-NH-), 3058, 2980, 2940 (CH, aliphatic), 1686 (>C=O), 1529 (-C=C-), 736 (-Ar-); 1H NMR (300 MHz, CDCl3, δ ppm): 1.45 (s, 9H, CH3), 4.53-4.55 (d, 2H, J = 6.3 Hz, CH2), 6.12 (brs, 1H, NH), 7.22-7.25 (m, 2H, Ar-H), 7.54-7.60 (m, 2H, Ar-H); HRMS (ESI): [M + H]+ calcd m/z 248.1400, found 248.1397.δ ppm): 1.45 (s, 9H, CH3), 4.53-4.55 (d, 2H, J = 6.3 Hz, CH2), 6.12 (brs, 1H, NH), 7.22-7.25 (m, 2H, Ar-H), 7.54-7.60 (m, 2H, Ar-H); HRMS (ESI): [M + H]+ calcd m/z 248.1400, found 248.1397.
пятница, 19 ноября 2010 г.
Motesanib
Motesanib
Targets: Motesanib (AMG-706) is a multitargeted anticancer agent with an inhibitory action on VEGFR1-3, PDGFR,c-KIT.
(WO/2002/066470) SUBSTITUTED ALKYLAMINE DERIVATIVES AND METHODS OF USE
четверг, 18 ноября 2010 г.
понедельник, 15 ноября 2010 г.
very interesting resource of chemical Internet
В анналах химического интернета обнаружил очень интересный ресурс
In the annals of the chemical Internet has found out very interesting resource
ttp://orgprepdaily.wordpress.com
In the annals of the chemical Internet has found out very interesting resource
ttp://orgprepdaily.wordpress.com
суббота, 13 ноября 2010 г.
Dasatinib
Dasatinib is an oral multi- BCR/ABL and Src family tyrosine kinases inhibitor approved for use in patients with chronic myelogenous leukemia (CML) after imatinib treatment and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). It is being evaluated for use in numerous other cancers, including advanced prostate cancer.
Name: N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide monohydrate
CAS No.: 302962-49-8
M.W.: 488.01
Formula: C22H26ClN7O2S
Synonym: BMS-354825, sprycel
Фармакологическое действие препарата Дазатиниб (Спрайсел)
Противоопухолевый препарат, мультикиназный ингибитор, наномолярных концентрациях ингибирует тирозинкиназы: BCR-ABL, семейство SRC (SRC, LCK, YES, FYN), c-KIT, EPHA2 и PDGFRβ. C помощью компьютерного моделирования установлено, что дазатиниб связывается со многими формами ABL киназы.
In vitro спрайсел показывает активность в различных лейкозных клеточных линиях, как чувствительных, так и резистентных к гливеку. Дазатиниб останавливает рост клеточных линий острого лимфобластного лейкоза и хронического миелолейкоза с гиперэкспрессией BCR-ABL. Дазатиниб показывает активность в случаях резистентность, связанную с мутациями BCR-ABL киназы, путем активацией альтернативных сигнальных путей, включающих киназы семейства SRC (LYN, НСК) и гиперэкспрессией гена лекарственной полирезистентности.
пятница, 12 ноября 2010 г.
Synthesis of Gefitinib from Methyl 3-Hydroxy-4-methoxybenzoate
Статья описывает все существующие варианты синтеза Gefinitib и предлагает новый.
Report describes all existing variants of synthesis Gefinitib and offers the new method.
http://www.mdpi.org/molecules/papers/12030673.pdf
Report describes all existing variants of synthesis Gefinitib and offers the new method.
http://www.mdpi.org/molecules/papers/12030673.pdf
четверг, 11 ноября 2010 г.
Palladia (toceranib)
Palladia
Пфайзер аннонсировал Палладию, как новое лекарственное средство для лечения рака у собак
Synthesis of Imatinib
(a) Zimmermann, J. EP Patent 564,409, 1993.
(b) Zimmermann, J. U.S. Patent 5,521,184, 1996.
(c) Zimmermann, J.; Buchdunger, E.;Mett, H.; Meyer, T.; Lydon, N. B.; Traxler, P. Bioorg. Med. Chem.Lett. 1996, 11, 1221.

Loiseleur, O.; Kaufmann, D.; Abel, S.; Buerger, H. M.; Meisenbach, M.; Schmitz, B.; Sedelmeier, G. W.O.Patent 03/066,613, 2003.
United States Patent 7674901
Process for preparation of imatinib base
An improved process for the preparation of imatinib base and its pharmaceutically acceptable acid addition salts by (a) reacting 2-methyl-5-nitroaniline with cyanamide in the presence of hydrochloric acid to obtain 1-(2-methyl-5-nitrophenyl)guanidine hydrochloride; (b) converting 1-(2-methyl-5-nitrophenyl)guanidine hydrochloride to 1-(2-methyl-5-nitrophenyl)guanidine nitrate; (c) condensing 3-acetylpyridine with N,N-dimethylformamide dimethyl acetal to obtain 3-(dimethylamino)-1-(3-pyridinyl)-prop-2-en-1-one; (d) reacting 3-(dimethylamino)-1-(3-pyridinyl)-prop-2-en-1-one with 1-(2-methyl-5-nitrophenyl)guanidine nitrate to obtain N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine; (e) reducing N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine using hydrazine in the presence of Raney nickel to obtain N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidine-amine; (f) condensing N-(5-amino-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidine-amine with 4-chloromethylbenzoyl chloride in the presence of an inorganic base to obtain 4-(chloromethyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)benzamide; and (g) condensing 4-(chloromethyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)benzamide with an excess of N-methylpiperazine to obtain imatinib base; and adding water or a mixture of water and an organic solvent; and isolating said imatinib base. The process allows for using simple starting materials, while simultaneously avoiding a laborious isolation and purification of intermediates and the final product, thereby facilitating scale-up.
The synthesis of Bcr-Abl inhibiting anticancer
pharmaceutical agents imatinib, nilotinib and dasatinib
Benjamin J. Deadman,a Mark D. Hopkin,a Ian R. Baxendaleb and Steven V. Ley*a
pharmaceutical agents imatinib, nilotinib and dasatinib
Benjamin J. Deadman,a Mark D. Hopkin,a Ian R. Baxendaleb and Steven V. Ley*a
Org. Biomol. Chem., 2013, Advance Article
среда, 10 ноября 2010 г.
Pelitinib
Name | (2E)-N-(4-((3-Chloro-4-fluorophenyl)amino)-7-ethoxyquinolin-6-yl)-3-cyano-4-(dimethylamino)but-2-enamide |
CAS | 257933-82-7 |
Formula | C24H23ClFN5O2 |
MW | 467.92 |
Synonim | EKB-569 |
Smile | Fc3ccc(Nc1c2cc(NC(=O)\C=C\CN(C)C)c(OCC)cc2ccc1C#N)cc3Cl |
Logp | |
TPSA | |
URL | |
Cancer Treat Rev. 2009, 5(8), 685-691 Biological Activity of Pelitinib: Pelitinib (EKB-569) малая молекула ряда 3-цианохинолина с потенциальной противораковой активностью. Pelitinib связывается ковалентно с рецептором эпидермального фактора роста (EGFR) ErbB-1,-2 и -4, тем самым запрещает фосфорилирование рецептора и передачу сигналов, в результате происходит подавление пролиферации и апоптоза в опухолевых клетках. Pelitinib препятствует EFR-индуцированному фосфорилированию EGF-R и росту опухолей, которые вызываются гиперэкспрессией EGF-R на животных моделях. | |
Pelitinib (EKB-569) series of small molecule 3-cyanoquinoline with potential anticancer activity. Binds covalently with Pelitinib retseptorfvb epidermal growth factor receptor (EGFR) ErbB-1, -2 and -4, thereby prevents receptor phosphorylation and signaling and resulting in the inhibition of proliferation and apoptosis in tumor cells. Pelitinib prevents EFR-induced phosphorylation of EGF-R and the growth of tumors that are caused by overexpression of EGF-R in animal models. Nature Reviews Cancer, 2010, vol 10, page 760-774 | |
Quizartinib
Name | 3-(4-(7-(2-morpholinoethoxy)-1-(5-(tert-Butyl)isoxazol-3-yl)benzo[d]imidazo[2,1-b]thiazol-2-yl)phenyl)urea |
CAS | 950769-58-1 (free base) 1132827-21-4 (2HCl) |
Formula | C29H32N6O4S |
MW | 560.7 |
Synonim | AC220, AC-010220 |
Smile | CC(C)(C)c6cc(NC(=O)Nc5ccc(c1cn4c(n1)sc3cc(OCCN2CCOCC2)ccc34)cc5)no6 |
Logp | 5.879 |
TPSA | 106.17 |
URL | http://www.ambitbio.com/quizartinib |
Quizartinib is a small molecule with potential anticancer activity. Quizartinib is a selective inhibitor of class III receptor tyrosine kinases, including FMS-related tyrosine kinase 3 (FLT3/STK1), stem cell factor receptor (SCFR / KIT), colony-stimulating factor 1 receptor (CSF1R/FMS) and platelet-derived growth factor receptors (PDGFRs .) Able to inhibition of ligand-independent cell proliferation and apoptosis. Mutations in FLT3 are the most frequent genetic alterations in acute myeloid leukemia (AML) and occur in approximately 30% of cases of AML. | |
Quizartinib представляет собой малую молекулу с потенциальной противораковой активностью. Quizartinib является селективным ингибитором класса III рецепторов тирозин киназ, в том числе FMS-связанных тирозинкиназы 3 (FLT3/STK1), фактор стволовых клеток рецепторов (SCFR / KIT), колониестимулирующий фактор 1 рецепторов (CSF1R/FMS) и тромбоцитарный рецепторов фактора роста (PDGFRs). Способен к торможению лиганд-независимой клеточной пролиферации и апоптоза. Мутации в FLT3 являются наиболее частыми генетическими изменениями в остром миелобластном лейкозе (ОМЛ) и встречаются примерно в 30% случаев ОМЛ. | |
Synthesis of quizartinib
Синтез куизартиниба
Подписаться на:
Комментарии (Atom)
Favorites chemistry resources
Обзоры
Ярлыки
AB1010
(1)
ABT-869
(1)
AG-013736
(1)
ALK-kinase
(1)
AMG-706
(1)
AP24534
(1)
AV-951
(1)
AZD-0530
(1)
AZD2171
(2)
Afatinib
(2)
Apatinib
(1)
Armala
(1)
BAY 73-4506
(1)
BIBW 2992
(1)
BMS-540215
(2)
Bafenitib
(1)
Bafetinib
(2)
Brivanib
(2)
CEP-701
(1)
CHIR258
(1)
CP690550
(1)
Cediranib
(2)
Crizotinib
(1)
Dasatinib
(2)
Dovitinib
(1)
Erlotinib
(1)
Foretinib
(1)
Gefinitib
(1)
Gefitinib
(2)
Gold reagent
(1)
HKI 272
(1)
INNO-406
(2)
Icotinib
(1)
Iressa
(1)
MLN 518
(1)
Masitinib
(1)
Mubritinib
(2)
NS-187
(2)
Nexavar
(2)
OSI-774
(1)
PF-02341066
(1)
PHA-739358
(1)
Palladia
(1)
Pazopanib
(2)
Pelitinib
(1)
Ponatinib
(1)
Recentin
(2)
Regorafenib
(1)
Ruxolitinib
(1)
SKI-606
(2)
Saracatinib
(2)
Silimitasertib
(1)
Sorafenib
(2)
Sorafenib tosylate
(1)
Sprycel
(2)
Sunitinib
(2)
Sunitinib Malate
(2)
Sutent
(2)
TKI-258
(1)
Tarceva
(2)
Tasocitinib
(1)
Tivozanib
(1)
Tovok
(2)
Vatalanib
(1)
Votrient
(2)
YN968D1
(1)
ZD6474
(1)
axinitib
(1)
bosutinib
(2)
canertinib
(1)
danusertib
(1)
gleevec
(1)
imatinib
(1)
lapatinib
(2)
lestaurtinib
(1)
linifanib
(1)
nilotinib
(1)
pyrazole
(1)
sc-202353
(1)
targeted agents
(1)
tasigna
(1)
toceranib
(1)
tykerb
(2)
tyverb
(1)
vandetanib
(1)
zactima
(1)
Бринатиниб
(1)
Гефитиниб
(1)
Кризотиниб
(1)
Маситиниб
(1)
Сунитиниб
(2)
Тарцева
(2)
Цедираниб
(1)
Эрлотиниб
(2)
акситиниб
(1)
ваталаниб
(1)
гливек
(1)
дазатиниб
(2)
данусертиб
(1)
зактима
(1)
икотиниб
(1)
карнетиниб
(1)
куизартиниб
(1)
лапатиниб
(1)
лекарства
(1)
лекарство
(1)
линифаниб
(1)
нексавар
(1)
понатиниб
(1)
руксолитиниб
(1)
сорафениб
(1)
спрайсел
(2)
сутент
(1)
тайверб
(1)
тандуниб
(1)
凡德他尼
(1)
尼罗替尼
(1)
阿西替尼
(1)